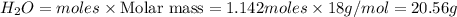Chemistry, 20.03.2021 08:40 leslieperez67
NH₄NO₃ → N₂O + 2H₂O When 45.70 g of NH₄NO₃ decomposes, what mass of each product is formed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1


Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
NH₄NO₃ → N₂O + 2H₂O When 45.70 g of NH₄NO₃ decomposes, what mass of each product is formed?...
Questions


Mathematics, 12.11.2021 09:00

History, 12.11.2021 09:00


History, 12.11.2021 09:10




Biology, 12.11.2021 09:40

Mathematics, 12.11.2021 09:40

Advanced Placement (AP), 12.11.2021 09:40

Mathematics, 12.11.2021 09:50


Mathematics, 12.11.2021 14:00

History, 12.11.2021 14:00

History, 12.11.2021 14:00

Business, 12.11.2021 14:00

Mathematics, 12.11.2021 14:00


Mathematics, 12.11.2021 14:00

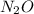 and 20.56 g of
and 20.56 g of 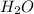 will be produced from 45.70 g of
will be produced from 45.70 g of 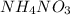
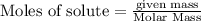
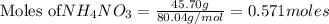
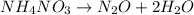
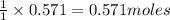 of
of 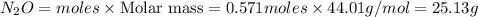
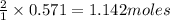 of
of