
Chemistry, 21.03.2021 02:10 jaclynnlyidaowdxnq
In a plot of ln(k) vs. 1/T, a slope of –4.3 x 102 K is obtained. What is the activation energy for this reaction?
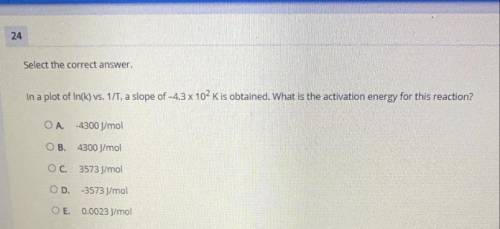

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
In a plot of ln(k) vs. 1/T, a slope of –4.3 x 102 K is obtained. What is the activation energy for t...
Questions


History, 22.06.2021 16:40

Mathematics, 22.06.2021 16:40





Mathematics, 22.06.2021 16:40



Mathematics, 22.06.2021 16:40


Mathematics, 22.06.2021 16:40

Mathematics, 22.06.2021 16:40

Biology, 22.06.2021 16:40

English, 22.06.2021 16:40


Mathematics, 22.06.2021 16:40

English, 22.06.2021 16:40



