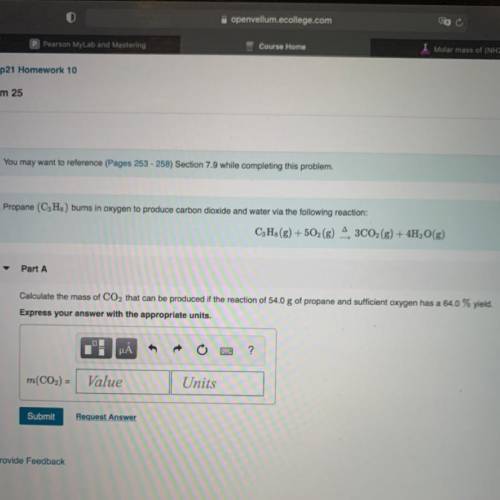
Chemistry, 21.03.2021 06:50 HTKPenguin
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient oxygen has a 64.0% yield.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

You know the right answer?
Calculate the mass of CO2 that can be produced if the reaction of 54.0 g of propane and sufficient o...
Questions


Mathematics, 08.11.2019 05:31



Physics, 08.11.2019 05:31

Social Studies, 08.11.2019 05:31

Mathematics, 08.11.2019 05:31


English, 08.11.2019 05:31



Arts, 08.11.2019 05:31




Geography, 08.11.2019 05:31

Mathematics, 08.11.2019 05:31

Social Studies, 08.11.2019 05:31