
Chemistry, 21.03.2021 07:30 Angelanova69134
How many moles of sodium will react with water to produce 2.0 mol of hydrogen in the following reaction? 2Na(s) + 2H20(1) - 2NaOH(aq) + H2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
How many moles of sodium will react with water to produce 2.0 mol of hydrogen in the following react...
Questions

Chemistry, 14.12.2021 03:20

History, 14.12.2021 03:20

Biology, 14.12.2021 03:20




Chemistry, 14.12.2021 03:20

Mathematics, 14.12.2021 03:20


Mathematics, 14.12.2021 03:20




Mathematics, 14.12.2021 03:20

Social Studies, 14.12.2021 03:20


Mathematics, 14.12.2021 03:20

History, 14.12.2021 03:20

History, 14.12.2021 03:20

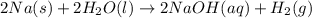
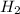 is produced by = 2 moles of
is produced by = 2 moles of 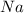
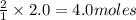 of
of 

