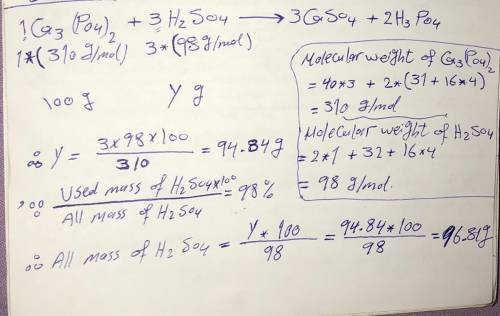Chemistry, 21.03.2021 18:40 kedjenpierrelouis
Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4.
What mass of concentrated H2SO4 (98% by mass) must be used to react completely
with 100.00 g of calcium phosphate?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Ca3(PO4)2 + 3H2SO4 3CaSO4 + 2H3PO4.
What mass of concentrated H2SO4 (98% by mass) must be used to...
Questions



English, 29.10.2020 03:20

Mathematics, 29.10.2020 03:20


Spanish, 29.10.2020 03:20

Mathematics, 29.10.2020 03:20

Biology, 29.10.2020 03:20

Mathematics, 29.10.2020 03:20

Physics, 29.10.2020 03:20

Biology, 29.10.2020 03:20



Biology, 29.10.2020 03:20


Mathematics, 29.10.2020 03:20

Mathematics, 29.10.2020 03:20


History, 29.10.2020 03:20