The first order decomposition of N2O5 to form NO2 and O2, has k =
s^-1 at 70°C.
What % o...

Chemistry, 21.03.2021 21:20 HOPING4632
The first order decomposition of N2O5 to form NO2 and O2, has k =
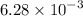
s^-1 at 70°C.
What % of the NO2 has decomposed after 278 s?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Questions



English, 05.03.2021 03:20

Mathematics, 05.03.2021 03:20

Chemistry, 05.03.2021 03:20

Health, 05.03.2021 03:20

Mathematics, 05.03.2021 03:20


Chemistry, 05.03.2021 03:20


Mathematics, 05.03.2021 03:20


Mathematics, 05.03.2021 03:20

Social Studies, 05.03.2021 03:20

Chemistry, 05.03.2021 03:20

Mathematics, 05.03.2021 03:20


Mathematics, 05.03.2021 03:20




