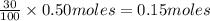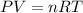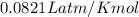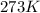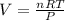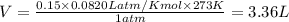Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
9 The Haber process is a reversible reaction. N2(g) + 3H2(g) 2NH3(g) The reaction has a 30% yield of...
Questions

Computers and Technology, 05.11.2020 18:50





History, 05.11.2020 18:50



Social Studies, 05.11.2020 18:50





World Languages, 05.11.2020 18:50


Business, 05.11.2020 18:50

Mathematics, 05.11.2020 18:50



Mathematics, 05.11.2020 18:50

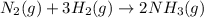
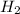 produce = 2 moles of
produce = 2 moles of 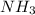
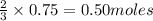 of
of