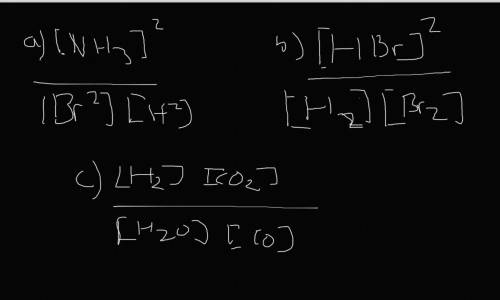Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Write the expressions for the equilibrium constants of the reactions below. (4 points each)
a. N2...
Questions

Biology, 25.02.2021 04:30


Mathematics, 25.02.2021 04:30





Social Studies, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30

English, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30


History, 25.02.2021 04:30

History, 25.02.2021 04:30


Mathematics, 25.02.2021 04:30