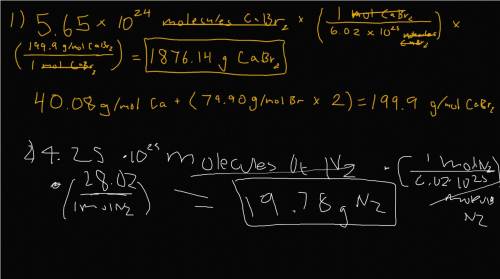Determine the mass of the following.
a) 5.65 x 1024 molecules of CaBr2
b) 4.25 x 1023 molecul...

Chemistry, 22.03.2021 01:50 elijahbravo7107
Determine the mass of the following.
a) 5.65 x 1024 molecules of CaBr2
b) 4.25 x 1023 molecules of N2
c) 9.10 x 1022 molecules of Ba(NO3)2
d) 3.01 x 1022 molecules of Ga2(CO3)3

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Questions

Mathematics, 24.06.2019 07:00




Mathematics, 24.06.2019 07:00

Chemistry, 24.06.2019 07:00


Advanced Placement (AP), 24.06.2019 07:00







Chemistry, 24.06.2019 07:00



Biology, 24.06.2019 07:00