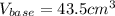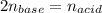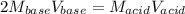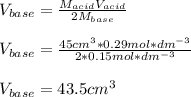Chemistry, 22.03.2021 02:10 juansantos7b
Calculate the volume of a 0.15 mol dm-3 Ba(OH)2 solution required to completely neutralize 45 cm3 of a 0.29 mol dm-3 HNO3 solution. Note: Ba(OH)2 + 2HNO3 --> Ba(NO3)2 + 2H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Calculate the volume of a 0.15 mol dm-3 Ba(OH)2 solution required to completely neutralize 45 cm3 of...
Questions

Mathematics, 16.09.2021 08:50


Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Chemistry, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

English, 16.09.2021 08:50


History, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50

Physics, 16.09.2021 08:50


Mathematics, 16.09.2021 08:50

Mathematics, 16.09.2021 08:50