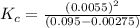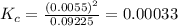Chemistry, 22.03.2021 20:10 xcrysttallx
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g) <-> 2I (g) When 0.095 M I2 is initially placed in a previously empty container and sealed, the system slowly reaches equilibrium. When equilibrium is reached, it is found that there is an equilibrium concentration of 0.0055 M of the monatomic form of iodine. Calculate the (unitless) equilibrium constant Kc. Round your answer to two sig figs, and express it in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
QUESTION 6 Consider the following reaction between the diatomic and monatomic forms of iodine: I2 (g...
Questions


English, 17.11.2020 04:50


Mathematics, 17.11.2020 04:50

English, 17.11.2020 04:50

Mathematics, 17.11.2020 04:50


English, 17.11.2020 04:50






English, 17.11.2020 04:50



Mathematics, 17.11.2020 04:50

Mathematics, 17.11.2020 04:50

Mathematics, 17.11.2020 04:50

Mathematics, 17.11.2020 04:50

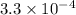
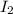 = 0.095 M
= 0.095 M 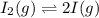
![K_c=\frac{[l]^2}{[I_2]}](/tpl/images/1211/7332/312cd.png)