
Chemistry, 22.03.2021 21:40 hardwick744
The solubility product for Zn(OH)2 is 3.0×10−16. The formation constant for the hydroxo complex, Zn(OH)2−4, is 4.6×1017. What is the minimum concentration of OH− required to dissolve 1.7×10−2 mol of Zn(OH)2 in a liter of solution?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
The solubility product for Zn(OH)2 is 3.0×10−16. The formation constant for the hydroxo complex, Zn(...
Questions

History, 27.08.2020 04:01


Social Studies, 27.08.2020 04:01





Mathematics, 27.08.2020 04:01

Physics, 27.08.2020 04:01






History, 27.08.2020 04:01


Mathematics, 27.08.2020 04:01



![[OH^-]= 1.12 \times 10^{-2} \ M](/tpl/images/1212/0889/be159.png)
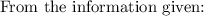
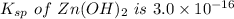
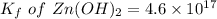
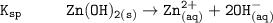
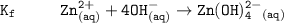
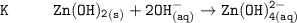
![K = \dfrac{Zn(OH)^-_4}{[OH^-]^2} \\ \\](/tpl/images/1212/0889/c5800.png)
![[OH^-]^2= \dfrac {0.017}{1.380 \times 10^2}](/tpl/images/1212/0889/33df3.png)
![[OH^-]^2=1.232 \times 10^{-4}](/tpl/images/1212/0889/f97e0.png)


