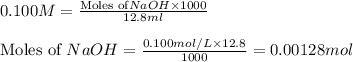Chemistry, 22.03.2021 22:30 jayden6467
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium hydroxide solution. The average volume of NaOH required to reach the endpoint was 12.8 mL. a. Calculate the number of moles of sodium hydroxide required to reach the endpoint. Show your work in equation editor. Remember to use units and report your answer to the proper number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2
You know the right answer?
molecular formula citric acid2.A 10.0 mL sample of pineapple juice was titrated with 0.100 M sodium...
Questions

Chemistry, 07.01.2020 10:31




Mathematics, 07.01.2020 10:31








Mathematics, 07.01.2020 10:31


Mathematics, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31


Mathematics, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31

Mathematics, 07.01.2020 10:31

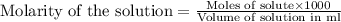
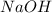 solution = 0.100 M
solution = 0.100 M