
Chemistry, 23.03.2021 01:30 Kathryn014
The boiling point of ch4 would be _ the boiling point of ch3ch2ch3
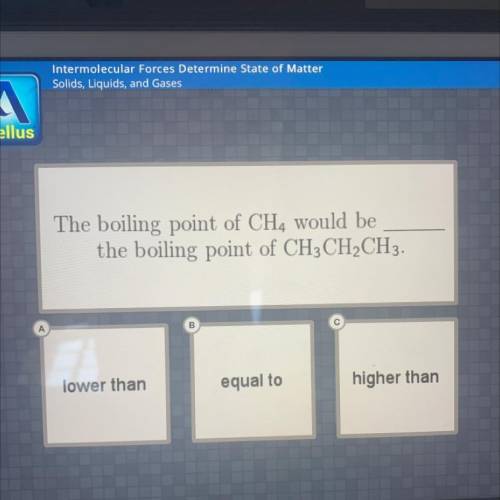

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
The boiling point of ch4 would be _ the boiling point of ch3ch2ch3
...
...
Questions




History, 19.10.2019 11:30







Physics, 19.10.2019 11:30





Geography, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30


History, 19.10.2019 11:30



