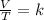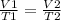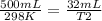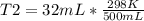Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
500mL of air are trapped in a tube over mercury at 25°C. It is found that, after six
days, the air...
Questions

Chemistry, 02.02.2021 15:20

Social Studies, 02.02.2021 15:20

Social Studies, 02.02.2021 15:20

Engineering, 02.02.2021 15:20


Chemistry, 02.02.2021 15:20

Social Studies, 02.02.2021 15:20

Mathematics, 02.02.2021 15:20

Chemistry, 02.02.2021 15:20

Business, 02.02.2021 15:20

Geography, 02.02.2021 15:20

Mathematics, 02.02.2021 15:20

History, 02.02.2021 15:20

English, 02.02.2021 15:20

Mathematics, 02.02.2021 15:20

Advanced Placement (AP), 02.02.2021 15:20

Mathematics, 02.02.2021 15:20

Geography, 02.02.2021 15:20

Mathematics, 02.02.2021 15:20

Business, 02.02.2021 15:20