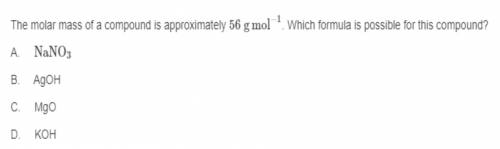Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3


Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
The molar mass of a compound is approximately 56 g mol ^-1. Which formula is possible for this compo...
Questions

Mathematics, 15.07.2019 16:40

Arts, 15.07.2019 16:40

History, 15.07.2019 16:40


Biology, 15.07.2019 16:40

Mathematics, 15.07.2019 16:40








Chemistry, 15.07.2019 16:40



Mathematics, 15.07.2019 16:40

Mathematics, 15.07.2019 16:40

Social Studies, 15.07.2019 16:40