
Chemistry, 23.03.2021 17:20 LarryJoeseph
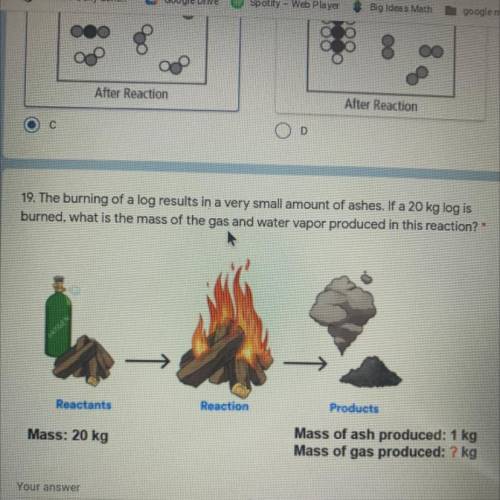
The burning of a log results in a very small amount of ashes. If a 20 kg log is
burned, what is the mass of the gas and water vapor produced in this reaction? *
OXYDEN
Reactants
Reaction
Products
Mass: 20 kg
Mass of ash produced: 1 kg
Mass of gas produced: ? kg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1


Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1

Chemistry, 23.06.2019 14:00
How are casts formed by decaying organisms? organisms turn into rock over time. organisms leave carbon residue on a rock. organisms leave impressions in sediment that hardens into rock. impressions left by organisms are filled in with sediment that hardens into rock.
Answers: 2
You know the right answer?
The burning of a log results in a very small amount of ashes. If a 20 kg log is
burned, what is the...
Questions



Mathematics, 01.07.2019 19:30


Biology, 01.07.2019 19:30


Social Studies, 01.07.2019 19:30






Mathematics, 01.07.2019 19:30


English, 01.07.2019 19:30

Health, 01.07.2019 19:30



English, 01.07.2019 19:30



