
Chemistry, 23.03.2021 18:10 rivasalejandro854
The pressure inside an aerosol can is 3.80 atm at 25.0°C. If the temperature is increased from 25.0°C to 100.0°C, what would be
the pressure inside the can?
A. 5.05 atm
B. 4.76 atm
C. 15.2 atm
D. 3.04 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
The pressure inside an aerosol can is 3.80 atm at 25.0°C. If the temperature is increased from 25.0°...
Questions

Social Studies, 13.11.2019 04:31

Mathematics, 13.11.2019 04:31


Medicine, 13.11.2019 04:31











Computers and Technology, 13.11.2019 04:31





Computers and Technology, 13.11.2019 04:31

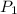 = Initial pressure = 3.8 atm
= Initial pressure = 3.8 atm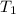 = Initial temperature =
= Initial temperature = 
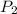 = Final pressure
= Final pressure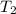 = Final temperature =
= Final temperature = 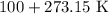
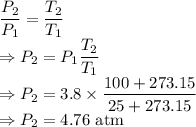
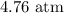 .
.

