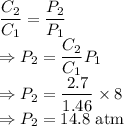Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
A gas has a solubility of 1.46 g L at 8.00 atm of pressure. What is the pressure of a sample of the...
Questions

Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00


Mathematics, 18.03.2021 19:00

Mathematics, 18.03.2021 19:00


English, 18.03.2021 19:00

Social Studies, 18.03.2021 19:00


Advanced Placement (AP), 18.03.2021 19:00

Arts, 18.03.2021 19:00

Computers and Technology, 18.03.2021 19:00




English, 18.03.2021 19:00


Mathematics, 18.03.2021 19:00

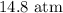
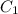 = Initial concentration = 1.46 g/L
= Initial concentration = 1.46 g/L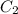 = Final concentration = 2.7 g/L
= Final concentration = 2.7 g/L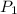 = Initial pressure = 8 atm
= Initial pressure = 8 atm = Final pressure
= Final pressure