
Chemistry, 23.03.2021 19:50 emily200705
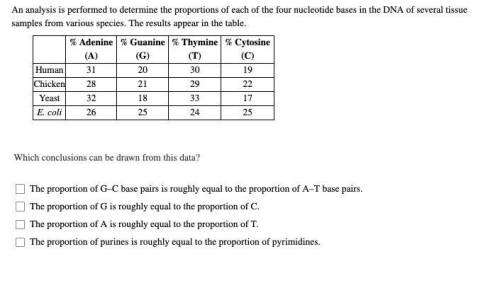
An analysis is performed to determine the proportions of each of the four nucleotide bases in the DNA of several tissue samples from various species. The results appear in the table. Which conclusions can be drawn from this data?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
An analysis is performed to determine the proportions of each of the four nucleotide bases in the DN...
Questions

Mathematics, 29.01.2021 17:40


Biology, 29.01.2021 17:40

Computers and Technology, 29.01.2021 17:40





Mathematics, 29.01.2021 17:40

Social Studies, 29.01.2021 17:40

Mathematics, 29.01.2021 17:40


Mathematics, 29.01.2021 17:40

Mathematics, 29.01.2021 17:50


Mathematics, 29.01.2021 17:50




Mathematics, 29.01.2021 17:50



