Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
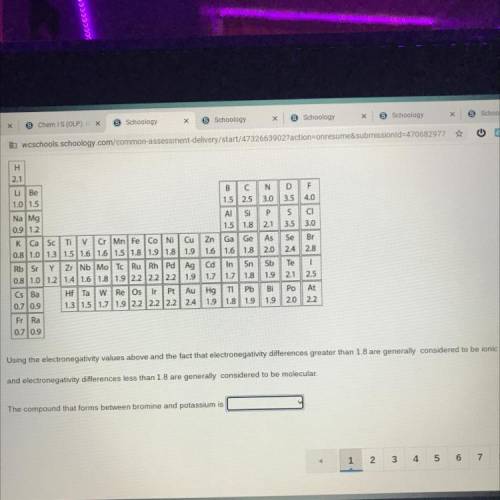
The compound that forms between bromine and potassium is
a. ionic
b. molecular/covalent...
a. ionic
b. molecular/covalent...
Questions

Advanced Placement (AP), 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50


Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50


Mathematics, 18.03.2021 16:50





Chemistry, 18.03.2021 16:50

English, 18.03.2021 16:50