
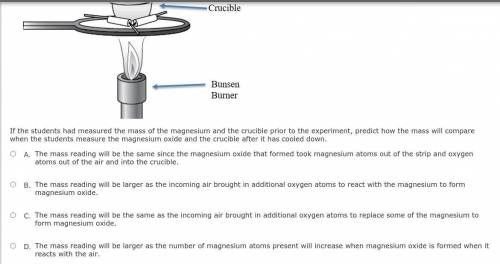
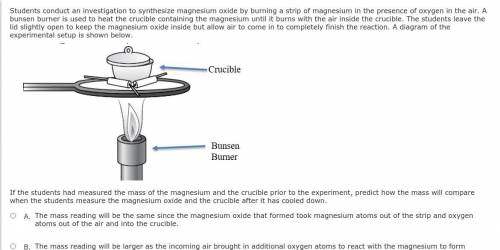
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in the presence of oxygen in the air. A bunsen burner is used to heat the crucible containing the magnesium until it burns with the air inside the crucible. The students leave the lid slightly open to keep the magnesium oxide inside but allow air to come in to completely finish the reaction. A diagram of the experimental setup is shown below.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Students conduct an investigation to synthesize magnesium oxide by burning a strip of magnesium in t...
Questions


Mathematics, 27.09.2020 01:01

Mathematics, 27.09.2020 01:01

Mathematics, 27.09.2020 01:01

Mathematics, 27.09.2020 01:01



Mathematics, 27.09.2020 01:01

Mathematics, 27.09.2020 01:01

Computers and Technology, 27.09.2020 01:01



Mathematics, 27.09.2020 01:01



Chemistry, 27.09.2020 01:01

History, 27.09.2020 01:01



Mathematics, 27.09.2020 01:01



