
Chemistry, 24.03.2021 20:30 oneicyahdaley10
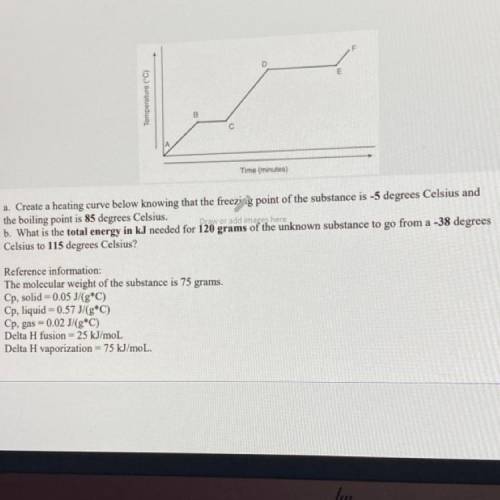
Create a heating curve below knowing that the freezing point of the substance is -5 degrees Celsius and
the boiling point is 85 degrees Celsius.
What is the total energy in kJ needed for 120 grams of the unknown substance to go from a -38 degrees
Celsius to 115 degrees Celsius?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

You know the right answer?
Create a heating curve below knowing that the freezing point of the substance is -5 degrees Celsius...
Questions

Mathematics, 29.03.2021 07:50




Mathematics, 29.03.2021 07:50

Mathematics, 29.03.2021 07:50



Chemistry, 29.03.2021 07:50

Mathematics, 29.03.2021 07:50

Chemistry, 29.03.2021 07:50


Biology, 29.03.2021 07:50



Chemistry, 29.03.2021 07:50

English, 29.03.2021 07:50


Mathematics, 29.03.2021 07:50

Mathematics, 29.03.2021 07:50



