
Chemistry, 25.03.2021 03:20 galaxicorn45
I've been stuck on this
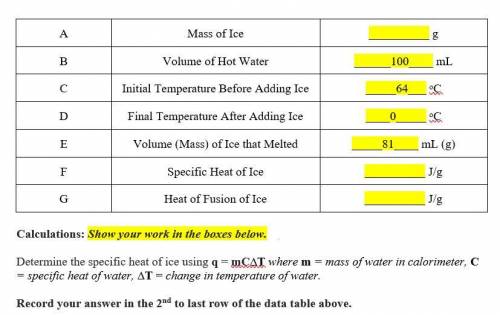
Determine the specific heat of ice using q = mC∆T where m = mass of water in calorimeter, C = specific heat of water, ∆T = change in temperature of water.
Determine the heat of fusion of ice using q = m∆Hf where q = heat, m = mass of substance being melted, ∆Hf = heat of fusion of substance being melted.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

You know the right answer?
I've been stuck on this
Determine the specific heat of ice using q = mC∆T where m = mass of water i...
Questions

History, 24.03.2021 01:20

Physics, 24.03.2021 01:20



English, 24.03.2021 01:20


History, 24.03.2021 01:20




Mathematics, 24.03.2021 01:20



Mathematics, 24.03.2021 01:20

Mathematics, 24.03.2021 01:20

Social Studies, 24.03.2021 01:20

Mathematics, 24.03.2021 01:30

Geography, 24.03.2021 01:30

History, 24.03.2021 01:30

Chemistry, 24.03.2021 01:30



