
Chemistry, 25.03.2021 03:00 lakhanir2013
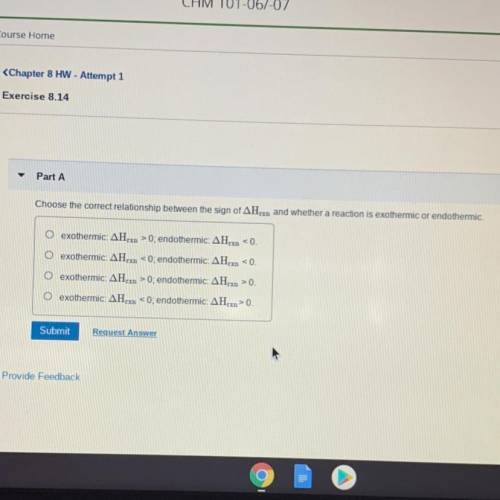
Choose the correct relationship between the sign of delta Hrxn and whether a reaction is exorhermic or endothermic.
•exothermic: delta Hrxn>0; endothermic: delta Hrxn<0.
•exothermic: delta Hrxn<0; endothermic: delta Hrxn<0.
•exothermic: delta Hrxn>0; endothermic: delta Hrxn>0.
•exothermic: delta Hrxn<0; endothermic: delta Hrxn>0.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 15:00
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b.liquid c.gas
Answers: 2

Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1

Chemistry, 23.06.2019 21:00
The two most abundant elements in earth's core are a. silicon and oxygen b. iron and magnesium c. nickel and silicon d. iron and nickel
Answers: 1
You know the right answer?
Choose the correct relationship between the sign of delta Hrxn and whether a reaction is exorhermi...
Questions



Business, 29.05.2021 23:10



Social Studies, 29.05.2021 23:10


History, 29.05.2021 23:10


Health, 29.05.2021 23:10

Mathematics, 29.05.2021 23:10

Mathematics, 29.05.2021 23:10

Mathematics, 29.05.2021 23:10


Mathematics, 29.05.2021 23:10





Mathematics, 29.05.2021 23:20



