
Chemistry, 25.03.2021 07:00 nauticatyson9
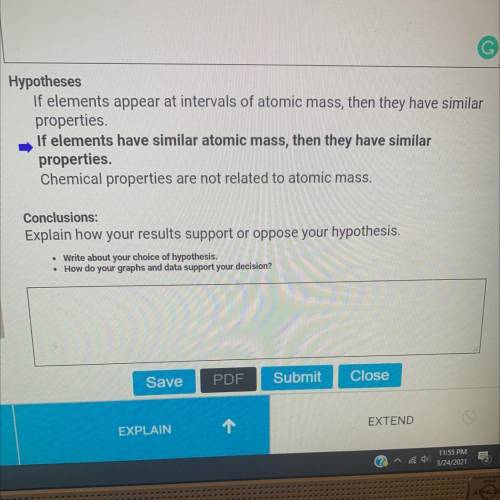
Which hypothesis is correct?
o If elements appear at intervals of atomic mass, then they have similar properties.
o If elements have similar atomic mass, then they have similar properties.
o Chemical properties are not related to atomic mass .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

You know the right answer?
Which hypothesis is correct?
o If elements appear at intervals of atomic mass, then they have simil...
Questions

Mathematics, 17.10.2021 01:00



Mathematics, 17.10.2021 01:00


Mathematics, 17.10.2021 01:00

Mathematics, 17.10.2021 01:00


Mathematics, 17.10.2021 01:00


Mathematics, 17.10.2021 01:00

Mathematics, 17.10.2021 01:00




Mathematics, 17.10.2021 01:00

English, 17.10.2021 01:00


Mathematics, 17.10.2021 01:00

Mathematics, 17.10.2021 01:00



