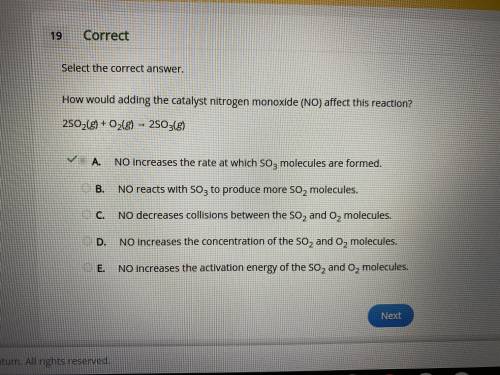
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A.
NO increases the rate at which SO3 molecules are formed.
B.
NO reacts with SO3 to produce more SO2 molecules.
C.
NO decreases collisions between the SO2 and O2 molecules.
D.
NO increases the concentration of the SO2 and O2 molecules.
E.
NO increases the activation energy of the SO2 and O2 molecules

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 12:30
How do you interpret a chromatogram for what mixtures contain?
Answers: 3

You know the right answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions

Mathematics, 06.12.2020 01:00





Physics, 06.12.2020 01:00

Mathematics, 06.12.2020 01:00


Mathematics, 06.12.2020 01:00

Chemistry, 06.12.2020 01:00

Chemistry, 06.12.2020 01:00

Mathematics, 06.12.2020 01:00

History, 06.12.2020 01:00


Mathematics, 06.12.2020 01:00


Biology, 06.12.2020 01:00


Biology, 06.12.2020 01:00

Health, 06.12.2020 01:00