These are the rest of the questions right down below
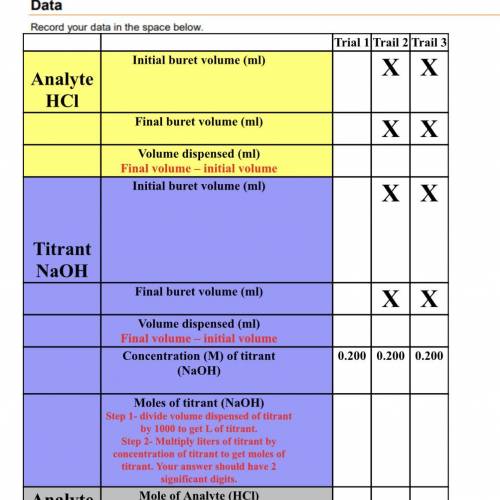
Analyte
HCl
Mole of Analyte (HCl)<...

These are the rest of the questions right down below
Analyte
HCl
Mole of Analyte (HCl)
(Equal to the moles of titrant)
Concentration (M)of analyte (HCl)
Step 1- divide volume dispensed of analyte by 1000 to get L of analyte
Step 2- Divide moles of analyte by liters of analyte to get concentration.
Average concentration(M) of analyte.
Add up the analyte concentrations from the three trials. Divide your answer by 3. Include 3 significant digits in your answer.
Percent error of concentration (M) of analyte.
Actual concentration of HCl = 0.120 M
Experimental concentration- Use the average you calculated.
Step 1- Subtract experimental value from actual value.
Step 2- Divide answer in Step 1 by actual value.
Step 3- Multiply answer in Step 3 by 100.
Your answer should be expressed as a percentage.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Questions


Mathematics, 20.07.2020 21:01





History, 20.07.2020 21:01



Mathematics, 20.07.2020 21:01

Spanish, 20.07.2020 21:01

Mathematics, 20.07.2020 21:01


Mathematics, 20.07.2020 21:01


Geography, 20.07.2020 21:01



Mathematics, 20.07.2020 21:01

Mathematics, 20.07.2020 21:01



