Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
❤️URGENT 10 EACH❤️ty
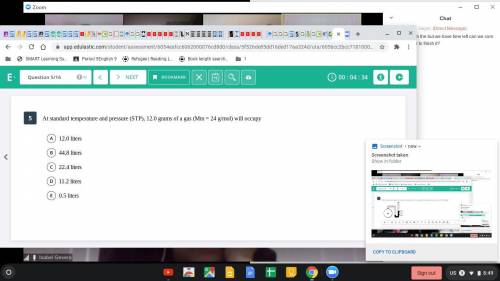
At standard temperature and pressure (STP), 12.0 grams of a gas (Mm=24 g/mol)...
Questions


Social Studies, 04.08.2019 21:30



History, 04.08.2019 21:30

Mathematics, 04.08.2019 21:30

Health, 04.08.2019 21:30







Health, 04.08.2019 21:30


Mathematics, 04.08.2019 21:30

History, 04.08.2019 21:30


Mathematics, 04.08.2019 21:30