
Chemistry, 25.03.2021 16:20 kyrajewell2016
What is the volume of 2.0 grams H2 if the temperature is 27 Celsius and the pressure is 0.82 atm?
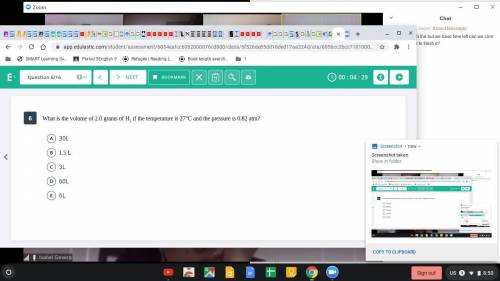

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
What is the volume of 2.0 grams H2 if the temperature is 27 Celsius and the pressure is 0.82 atm?
Questions




Mathematics, 06.10.2019 09:00


History, 06.10.2019 09:00


History, 06.10.2019 09:00

Computers and Technology, 06.10.2019 09:00











Computers and Technology, 06.10.2019 09:00



