
Chemistry, 25.03.2021 16:30 kirkhester1
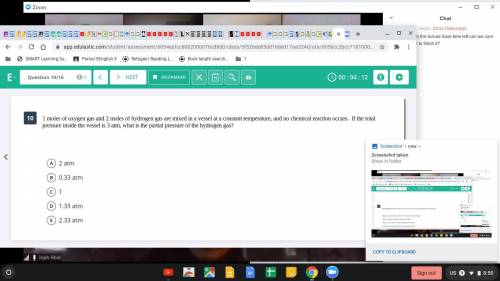
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no chemical reaction occurs. If the total pressure inside the vessel is 3 atm, what is the partial pressure of the hydrogen gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no...
Questions

Mathematics, 18.06.2020 23:57


History, 18.06.2020 23:57


Biology, 18.06.2020 23:57



Geography, 18.06.2020 23:57




Computers and Technology, 18.06.2020 23:57

Mathematics, 18.06.2020 23:57










