
Chemistry, 25.03.2021 18:20 phebusadrian01
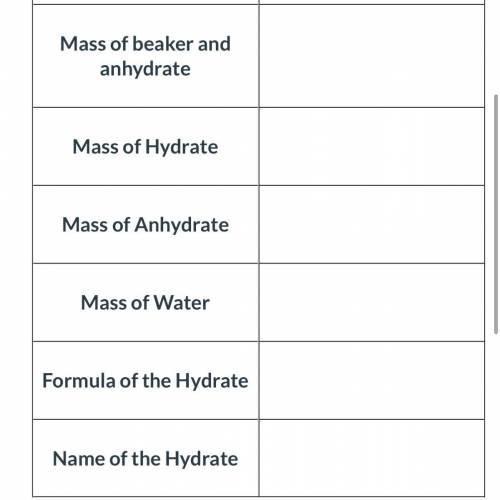
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to the beaker. When the beaker and hydrate was weighed again, the new mass was 62.29 grams. The beaker and the hydrated compound were heated and cooled several times to remove all of the water. The beaker and the anhydrate were then weighed and its new mass was determined to be 59.29 grams.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to...
Questions




Physics, 08.09.2020 14:01


Mathematics, 08.09.2020 14:01


Mathematics, 08.09.2020 14:01



Chemistry, 08.09.2020 14:01

Mathematics, 08.09.2020 14:01

History, 08.09.2020 14:01

Social Studies, 08.09.2020 14:01


Chemistry, 08.09.2020 14:01


Computers and Technology, 08.09.2020 14:01

Mathematics, 08.09.2020 14:01



