
Chemistry, 25.03.2021 18:30 Britny2386
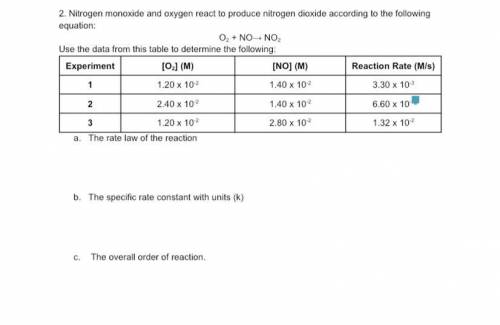
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:
The rate law of the reaction
The specific rate constant with units (k)
The overall order of reaction.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 13:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

You know the right answer?
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:...
Questions


Mathematics, 24.08.2020 14:01


Biology, 24.08.2020 14:01


English, 24.08.2020 14:01


Chemistry, 24.08.2020 14:01


Computers and Technology, 24.08.2020 14:01

History, 24.08.2020 14:01




Mathematics, 24.08.2020 14:01



Arts, 24.08.2020 14:01

Mathematics, 24.08.2020 14:01



