The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
<...

Chemistry, 25.03.2021 22:10 iesps010411
The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
Ag+ (aq) + e^- -> Ag(s)
Al(s) -> Al^3+ (aq) + 3e^-
What is the coefficient of silver in the final, balanced equation for this reaction?
A. 1
B. 2
C. 3
D. 4
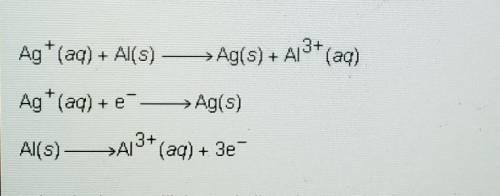

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Questions


Arts, 14.04.2021 16:30

Mathematics, 14.04.2021 16:30



Mathematics, 14.04.2021 16:30

Mathematics, 14.04.2021 16:30


Mathematics, 14.04.2021 16:30

Mathematics, 14.04.2021 16:30

Mathematics, 14.04.2021 16:30



Mathematics, 14.04.2021 16:30


Mathematics, 14.04.2021 16:30

Mathematics, 14.04.2021 16:30


Chemistry, 14.04.2021 16:30



