
Chemistry, 26.03.2021 07:10 21hendlill
4NH3 + 502 --> 4NO + 6H20
How much excess reactant is leftover after if 6.30g of ammonia react with
1.80g of oxygen?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 14:00
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2

You know the right answer?
4NH3 + 502 --> 4NO + 6H20
How much excess reactant is leftover after if 6.30g of ammonia react w...
Questions


English, 09.02.2021 17:10

Mathematics, 09.02.2021 17:10




Arts, 09.02.2021 17:10

Mathematics, 09.02.2021 17:10

Mathematics, 09.02.2021 17:10



Mathematics, 09.02.2021 17:10



French, 09.02.2021 17:10

Computers and Technology, 09.02.2021 17:10



English, 09.02.2021 17:10

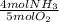 = 0.045 mol NH₃
= 0.045 mol NH₃

