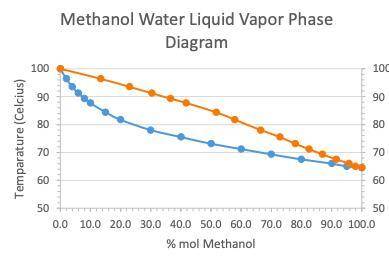
A student performed simple distillation on a 40:60mixture of Methanol and water (%
mol).
a....

Chemistry, 26.03.2021 08:10 josmanu235
A student performed simple distillation on a 40:60mixture of Methanol and water (%
mol).
a. At what temperature will the mixture boil?
b. What is the composition of the liquid collected from simple distillation?
2. Another student performed a fractional distillation on the same mixture of 40:60 (%
mol) Methanol/water mixture and found the liquid collected to contain 4% mol of
water.
a. At what temperature did the mixture containing 4% mol of water boil?
b. How many theoretical plates did the fractionating column used in this experiment
have?
c. What would be the minimum number of theoretical plates required to achieve
complete separation of the 40:60 (% mol) methanol-water mixture?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Questions

Mathematics, 17.10.2020 07:01

History, 17.10.2020 07:01

Mathematics, 17.10.2020 07:01

Mathematics, 17.10.2020 07:01

Mathematics, 17.10.2020 07:01


History, 17.10.2020 07:01


Mathematics, 17.10.2020 07:01



Health, 17.10.2020 07:01




Biology, 17.10.2020 07:01

History, 17.10.2020 07:01


Mathematics, 17.10.2020 07:01

History, 17.10.2020 07:01



