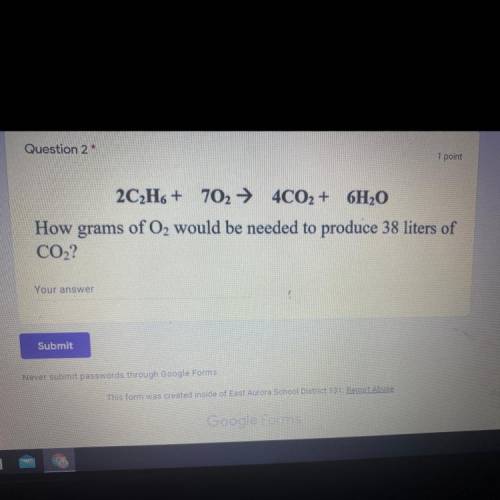
2C2H6 + 702 + 4CO2 + 6H2O
How grams of O2 would be needed to produce 38 liters of
CO2?
<...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Questions



History, 04.12.2020 20:00




Arts, 04.12.2020 20:00

History, 04.12.2020 20:00




Chemistry, 04.12.2020 20:00


Biology, 04.12.2020 20:00



Biology, 04.12.2020 20:00

Law, 04.12.2020 20:00


History, 04.12.2020 20:00