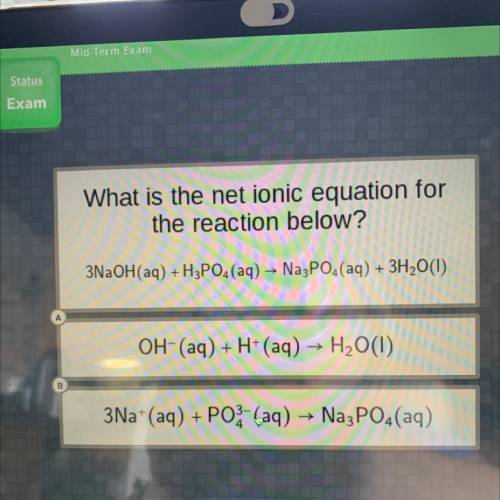
What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3...

Chemistry, 26.03.2021 20:40 afolmar2006
What is the net ionic equation for
the reaction below?
3NaOH(aq) +H3PO4 (aq) → Na3PO4(aq) + 3H2O(1)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Questions


Mathematics, 04.03.2021 02:00

Geography, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00





Mathematics, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00

Biology, 04.03.2021 02:00

Health, 04.03.2021 02:00

Mathematics, 04.03.2021 02:00


Mathematics, 04.03.2021 02:00

Health, 04.03.2021 02:00



