Chemistry, 26.03.2021 21:30 ariellake8551
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g) +2H2(g)Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, water, nitrogen monoxide, and hydrogen has the following composition: compound pressure at equilibrium N2 0.25 M H20 1.3 M NO 0.33 M H2 1.2 MCalculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g)...
Questions

Mathematics, 22.09.2021 14:30



Social Studies, 22.09.2021 14:30

Mathematics, 22.09.2021 14:30

Mathematics, 22.09.2021 14:40

English, 22.09.2021 14:40

Mathematics, 22.09.2021 14:40


Mathematics, 22.09.2021 14:40


Arts, 22.09.2021 14:40

Geography, 22.09.2021 14:40


Mathematics, 22.09.2021 14:40

Social Studies, 22.09.2021 14:40

Chemistry, 22.09.2021 14:40

Mathematics, 22.09.2021 14:40


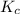

![K_c=\frac{[NO]^2\times [H_2]^2}{[H_2O]^2\times [N_2]^1}](/tpl/images/1224/3364/cca59.png)