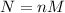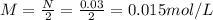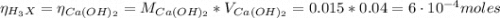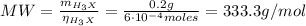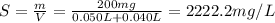Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1


Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 07:00
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
Calculate the molecular weight of 0.2g H3X solution if 50ml of this acid neutralized with 40ml of 0....
Questions


Mathematics, 09.09.2020 02:01

Mathematics, 09.09.2020 02:01


Mathematics, 09.09.2020 02:01




Business, 09.09.2020 02:01

Mathematics, 09.09.2020 02:01








Physics, 09.09.2020 02:01