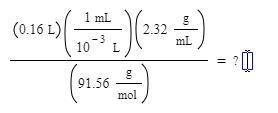(0.16L) (1mL/10^-3L) (2.32g/mL)/ (91.56 g/mol)
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
Questions


Mathematics, 21.01.2020 22:31


Biology, 21.01.2020 22:31

Mathematics, 21.01.2020 22:31

History, 21.01.2020 22:31


Mathematics, 21.01.2020 22:31

Biology, 21.01.2020 22:31


Social Studies, 21.01.2020 22:31


Biology, 21.01.2020 22:31

Biology, 21.01.2020 22:31


Mathematics, 21.01.2020 22:31


Physics, 21.01.2020 22:31

Biology, 21.01.2020 22:31

Chemistry, 21.01.2020 22:31