
Rank the following carboxylic acids by acid strength, with the strongest at the top and the weakest at the bottom. It may help to draw each Lewis structure. Drag and drop options into correct order. For keyboard navigation...SHOW MORE Press space or enter to grab A. CF3CO2H B. CHF2CO2HC. CH2FCO2H D. CH3CO2H

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Rank the following carboxylic acids by acid strength, with the strongest at the top and the weakest...
Questions

Computers and Technology, 28.06.2019 19:30


Biology, 28.06.2019 19:30



Mathematics, 28.06.2019 19:30


History, 28.06.2019 19:30

Health, 28.06.2019 19:30

English, 28.06.2019 19:30



Mathematics, 28.06.2019 19:30

History, 28.06.2019 19:30



Chemistry, 28.06.2019 19:30

Social Studies, 28.06.2019 19:30

Mathematics, 28.06.2019 19:30

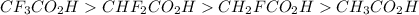
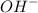 groups in the COOH group towards itself, making it relatively easy to loose the proton of the carboxyl group.
groups in the COOH group towards itself, making it relatively easy to loose the proton of the carboxyl group.

