

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Acid spills are often neutralized with sodium carbonate. For example
Na CO2 (s) + H2SO4 (aq) → Na2S...
Questions


Mathematics, 05.05.2020 10:07

Biology, 05.05.2020 10:08

Mathematics, 05.05.2020 10:08


English, 05.05.2020 10:08


Mathematics, 05.05.2020 10:08

Mathematics, 05.05.2020 10:08



Mathematics, 05.05.2020 10:08

History, 05.05.2020 10:08


Mathematics, 05.05.2020 10:08

History, 05.05.2020 10:08

Mathematics, 05.05.2020 10:08

History, 05.05.2020 10:08

History, 05.05.2020 10:08

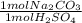 = 45 mol NaCO₂
= 45 mol NaCO₂


