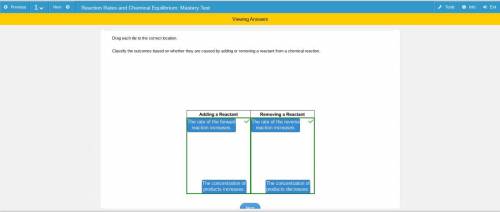
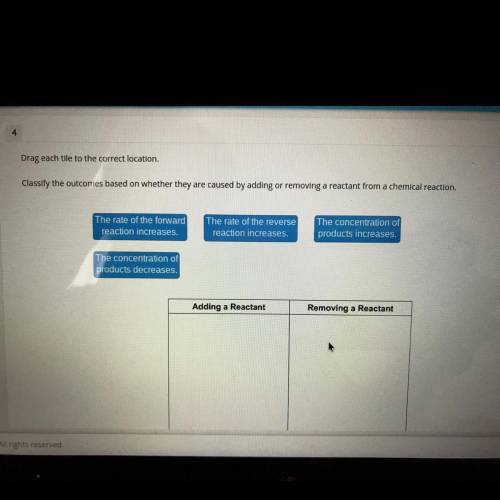
Drag each tile to the correct location.
Classify the outcomes based on whether they are caused by adding or removing a reactant from a chemical reaction.
The rate of the forward
reaction increases.
The rate of the reverse
reaction increases.
The concentration of
products increases.
The concentration of
products decreases.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

You know the right answer?
Drag each tile to the correct location.
Classify the outcomes based on whether they are caused by a...
Questions


Mathematics, 31.10.2020 01:00



Mathematics, 31.10.2020 01:00


Health, 31.10.2020 01:00


Spanish, 31.10.2020 01:00


English, 31.10.2020 01:00



Mathematics, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00

World Languages, 31.10.2020 01:00

Business, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00