
Chemistry, 28.03.2021 14:00 titalili0204
A water solution containing an unknown quantity of a
nonelectrolyte solute is found to have a freezing point of
–0.23°C. What is the molal concentration of the solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
A water solution containing an unknown quantity of a
nonelectrolyte solute is found to have a freez...
Questions

English, 29.07.2021 08:40



Mathematics, 29.07.2021 08:40


Mathematics, 29.07.2021 08:40

SAT, 29.07.2021 08:40



Mathematics, 29.07.2021 08:40

Mathematics, 29.07.2021 08:40

Mathematics, 29.07.2021 08:40

History, 29.07.2021 08:40

Chemistry, 29.07.2021 08:40



Mathematics, 29.07.2021 08:40

Social Studies, 29.07.2021 08:50

Social Studies, 29.07.2021 08:50

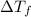 =
=  · b·i
· b·i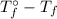
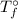 = The freezing point of pure water = 0°C
= The freezing point of pure water = 0°C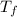 = The freezing point of the solution = -0.23°C
= The freezing point of the solution = -0.23°C

