
Chemistry, 28.03.2021 19:20 gwendallinesikes
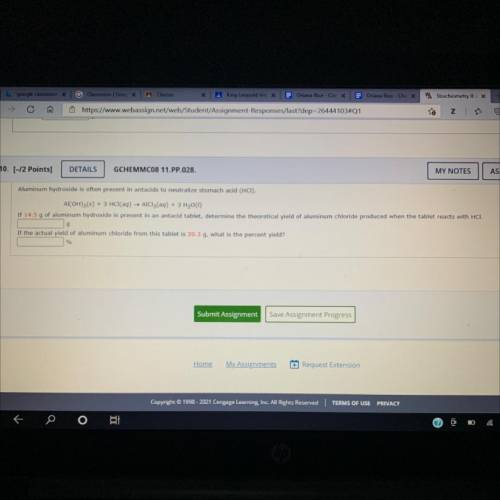
Aluminum hydroxide is often presant in antacids to neutralize stomach (HCI) Al(OH) 3 (s)+3HCl(aq) AlCl 3 (aq)+3H 2 O(l) is present in an antacid tablet, the theoretical yield of aluminum chloride produced the tablet reacts with HCH the actual yield of aluminum chloride from this tablet what the percent yield? SOMEONE PLEASE HELP!!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Aluminum hydroxide is often presant in antacids to neutralize stomach (HCI) Al(OH) 3 (s)+3HCl(aq) Al...
Questions

History, 31.12.2019 18:31

Mathematics, 31.12.2019 18:31

Mathematics, 31.12.2019 18:31

History, 31.12.2019 18:31

Chemistry, 31.12.2019 18:31

Mathematics, 31.12.2019 18:31


Computers and Technology, 31.12.2019 18:31


Mathematics, 31.12.2019 18:31


Biology, 31.12.2019 18:31

Social Studies, 31.12.2019 18:31

Physics, 31.12.2019 18:31




Computers and Technology, 31.12.2019 18:31


Mathematics, 31.12.2019 18:31



