
Chemistry, 28.03.2021 21:00 powellkhalil58
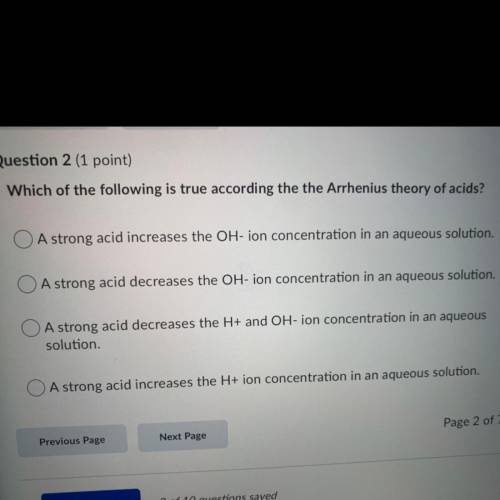
Which of the following is true according the the Arrhenius theory of acids?
A strong acid increases the OH-ion concentration in an aqueous solution.
A strong acid decreases the OH-ion concentration in an aqueous solution.
O A strong acid decreases the H+ and OH-ion concentration in an aqueous
solution.
A strong acid increases the H+ ion concentration in an aqueous solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Which of the following is true according the the Arrhenius theory of acids?
A strong acid increases...
Questions

Mathematics, 03.11.2020 03:00

Mathematics, 03.11.2020 03:00

Mathematics, 03.11.2020 03:00

Physics, 03.11.2020 03:00

English, 03.11.2020 03:00


Chemistry, 03.11.2020 03:00

Arts, 03.11.2020 03:00



English, 03.11.2020 03:00

Mathematics, 03.11.2020 03:00





History, 03.11.2020 03:00

Mathematics, 03.11.2020 03:00

Social Studies, 03.11.2020 03:00



