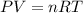Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

You know the right answer?
Calculate the volume occupied by 2.00 moles of H2 at 300.0 K and 1.25
atm...
atm...
Questions

English, 14.04.2021 14:00


History, 14.04.2021 14:00

English, 14.04.2021 14:00





English, 14.04.2021 14:00

Chemistry, 14.04.2021 14:00

Biology, 14.04.2021 14:00

Chemistry, 14.04.2021 14:00

Biology, 14.04.2021 14:00

Computers and Technology, 14.04.2021 14:00

Chemistry, 14.04.2021 14:00

Social Studies, 14.04.2021 14:00

Physics, 14.04.2021 14:00

Social Studies, 14.04.2021 14:00

Biology, 14.04.2021 14:00

Computers and Technology, 14.04.2021 14:00