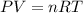Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
Calculate the volume occupied by 2.00 moles of H₂ at 300.0 K and 1.25 atm...
Questions

English, 11.10.2021 03:20


History, 11.10.2021 03:20

Chemistry, 11.10.2021 03:20

English, 11.10.2021 03:20


Mathematics, 11.10.2021 03:20

Mathematics, 11.10.2021 03:20

Computers and Technology, 11.10.2021 03:20

Mathematics, 11.10.2021 03:20

Mathematics, 11.10.2021 03:20


Social Studies, 11.10.2021 03:30


Computers and Technology, 11.10.2021 03:30



History, 11.10.2021 03:30

English, 11.10.2021 03:30