
Chemistry, 29.03.2021 17:20 elijahjacksonrp6z2o7
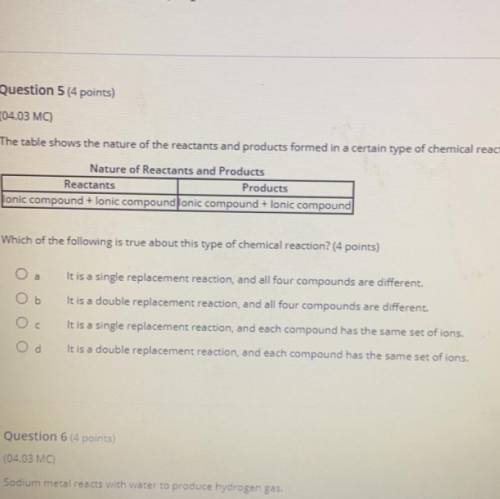
The table shows the nature of the reactants and products formed in a certain type of chemical reaction.
Nature of Reactants and Products
Reactants
Products
lonic compound + lonic compound lonic compound + lonic compound
Which of the following is true about this type of chemical reaction? (4 points)
It is a single replacement reaction, and all four compounds are different.
Ob
It is a double replacement reaction, and all four compounds are different.
It is a single replacement reaction, and each compound has the same set of ions.
Od
It is a double replacement reaction, and each compound has the same set of ions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
The table shows the nature of the reactants and products formed in a certain type of chemical reacti...
Questions


Mathematics, 19.12.2019 19:31

Mathematics, 19.12.2019 19:31



Mathematics, 19.12.2019 19:31

Mathematics, 19.12.2019 19:31


Mathematics, 19.12.2019 19:31

Mathematics, 19.12.2019 19:31


Spanish, 19.12.2019 19:31



History, 19.12.2019 19:31

English, 19.12.2019 19:31






